Recombinant Human Furin (carrier-free) 100 µg
Produit ni repris ni échangé excepté en cas d’erreur du prestataire.
Points clés
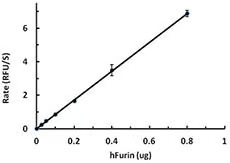
Furin belongs to the family of highly specific, calcium-dependent proprotein/prohormone convertases (PCs). It is expressed as a 794-amino-acid type-I transmembrane protein that is found in all vertebrates and many invertebrates. Furin and the other PCs contain prodomains that are flanked by the signal peptidase cleavage site on the amino-terminal side and by a conserved set of basic amino acids that comprise the autoproteolytic cleavage site on the carboxy-terminal side. The 83-amino-acid furin prodomain acts as an intramolecular chaperone that guides the folding and activation of the endoprotease. The release of furin's propeptide fragments by autocatalysis unmasks its endoprotease activity and enables it to cleave substrates where it is localized. The consensus site that furin cleaves is positioned after the carboxy-terminal arginine (Arg) residue in the sequence -Arg-X-Lys/Arg-Arg^- (where X is any amino acid and ^ identifies the cleavage site). Furin localizes to the Trans-Golgi Network (TGN). From the TGN, furin follows a highly regulated trafficking itinerary through several TGN/endosomal compartments and the cell surface. Some of the unique furin substrates include TGF-β-like precursors such as BMP10 during embryonic development, insulin-like growth factor-1 (IGF-1), membrane-type matrix metalloproteinases, and the iron-regulating protein hepcidin. Infectious agents also utilize furin-like cleavage as a requisite step of their infectious cycle. Numerous bacterial toxins are activated by furin-like cleavage on the cell surface, as occurs for anthrax toxin, or during their endocytic entry, as occurs for Pseudomonas exotoxin. Furin-like cleavage of some viral envelope proteins, including those of avian influenza virus, HIV-1 and measles virus, is required for infectious virion assembly. Furin is upregulated in several cancers, including non-small-cell lung carcinomas, squamous cell carcinomas of the head and neck, and glioblastoma.;
Garantie
Garantie 0 Mois
Description
Furin belongs to the family of highly specific, calcium-dependent proprotein/prohormone convertases (PCs). It is expressed as a 794-amino-acid type-I transmembrane protein that is found in all vertebrates and many invertebrates. Furin and the other PCs contain prodomains that are flanked by the signal peptidase cleavage site on the amino-terminal side and by a conserved set of basic amino acids that comprise the autoproteolytic cleavage site on the carboxy-terminal side. The 83-amino-acid furin prodomain acts as an intramolecular chaperone that guides the folding and activation of the endoprotease. The release of furin's propeptide fragments by autocatalysis unmasks its endoprotease activity and enables it to cleave substrates where it is localized. The consensus site that furin cleaves is positioned after the carboxy-terminal arginine (Arg) residue in the sequence -Arg-X-Lys/Arg-Arg^- (where X is any amino acid and ^ identifies the cleavage site). Furin localizes to the Trans-Golgi Network (TGN). From the TGN, furin follows a highly regulated trafficking itinerary through several TGN/endosomal compartments and the cell surface. Some of the unique furin substrates include TGF-β-like precursors such as BMP10 during embryonic development, insulin-like growth factor-1 (IGF-1), membrane-type matrix metalloproteinases, and the iron-regulating protein hepcidin. Infectious agents also utilize furin-like cleavage as a requisite step of their infectious cycle. Numerous bacterial toxins are activated by furin-like cleavage on the cell surface, as occurs for anthrax toxin, or during their endocytic entry, as occurs for Pseudomonas exotoxin. Furin-like cleavage of some viral envelope proteins, including those of avian influenza virus, HIV-1 and measles virus, is required for infectious virion assembly. Furin is upregulated in several cancers, including non-small-cell lung carcinomas, squamous cell carcinomas of the head and neck, and glioblastoma.;
Caractéristiques
- Fournisseur
- BioLegend Europe BV
- Marque
- BIOLEGEND
- Référence fabricant
- 719406
- Référence distributeur
- 719406
- Vendu par
- 100 μg
- Quantité
- N/A
- Lieu de fabrication
- USA
- Lieu de stockage
- Pays-Bas ou USA
- Soumis à carboglace
- non
- Classement dans le catalogue fournisseur
- Recombinant Protein
- Certification
- RUO
- Type d’application
- culture cellulaire
- Type de produit
- protéine
- Température de conservation (°C)
- -20 ou -70 °C
- Température de transport
- Blue Ice
- Organisme cible
- Human
- Source biologique
- CHO cells
- Seuil de coupure des masses moléculaires MWCO
- The mature 621 amino acid recombinant protein has a predicted molecular mass of approximately 67.1 kDa. The non-reduced and DTT-reduced proteins migrate at 65 - 75 and 70 - 80 kDa, respectively, by SDS-PAGE. Da
- Concentration
- 10 and 25 µg sizes are bottled at 200 µg/mL. 100 µg size and larger sizes are lot-specific and bottled at the concentration indicated on the vial. To obtain lot-specific concentration, please enter the lot number in our online tools.
- Pureté
- >90%, as determined by Coomasie stained SDS-PAGE. %
- Matière dangereuse
- Non
- Code douanier
- 38220000
- Classement NCBI
- 5045
- Nomenclature Nacres
- NA.77
- Nomenclature CEA
- SGP01
- Nomenclature IRSN
- 273
- Nomenclature INSERM
- NA.NA77
- Nomenclature CNRS
- NA77
- Nomenclature CHU
- 18.551
- Nomenclature DGOS
- LD11AOOO
- Type d'échantillon
- culture cellulaire
- Reprise en cas d’erreur client
- non